For life sciences organizations, balancing regulatory requirements, quality management, and cutting-edge R&D with the agility, innovation, and cost savings of the cloud is key to maintaining a competitive advantage. Aligning Enterprise Information Management (EIM) solutions with business priorities helps organizations achieve their strategic goals.

Balancing Innovation & Compliance in Life Sciences
For life sciences organizations, balancing regulatory requirements, quality management, and cutting-edge R&D with the agility, innovation, and cost savings of the cloud is key to maintaining a competitive advantage. Aligning Enterprise Information Management (EIM) solutions with business priorities helps organizations achieve their strategic goals.
What is the value of EIM to the Life Sciences industry?
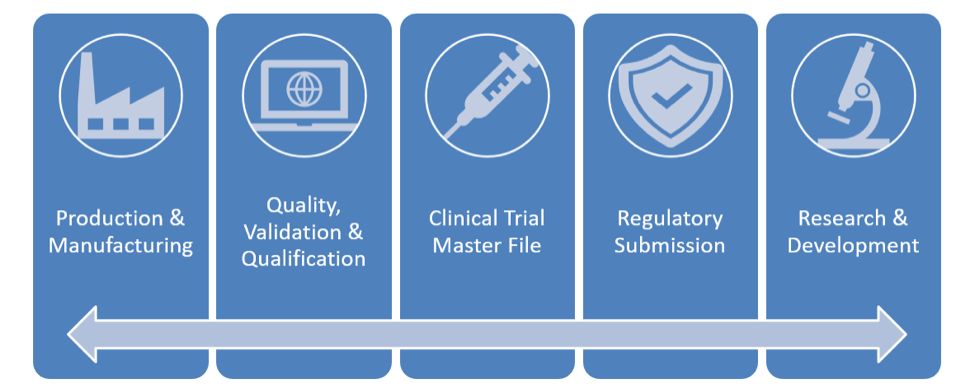
EIM alignment helps Life Sciences organizations bring safe, high-quality drugs and medical devices to market faster and at a lower cost by seamlessly sharing content and information throughout the drug lifecycle. It unifies processes that extend across domains, internal divisions, and external partners. By linking and sharing documents, it helps to 1) speed study startups, 2) manage regulatory submissions, and 3) govern quality and manufacturing processes with purpose-built solutions that leverage industry guidance and best practices.
For example, when it comes to production and manufacturing, EIM solutions provide comprehensive, predefined document taxonomies, lifecycles, and workflows tailored specifically for pharmaceutical and medical device products. These solutions have been pre-built to reduce time to market and ensure adherence to industry standards.
The same can be said for quality and validation processes, where EIM solutions help ensure compliance with current GMP standards through industry-specific data models, audit trails, reporting and e-signature support.
Moving beyond production and operations to areas like clinical trials, EIM can help to effectively plan, collect, and maintain essential documentation. With the challenges and risks of managing clinical data, electronic Trial Master File (eTMF) solutions help organizations control and synchronize study artifacts and track the progress of collecting clinical trial documentation. Such solutions reduce the complexity and risk for sponsors and CROs, while ensuring fast, secure access to documentation during and after trials.
And finally, with R&D and regulatory submissions, EIM solutions not only accelerate the submission process with built-in compliance and global control of content, but they also simplify the search and retrieval of archived submissions and their associated correspondence.
In each case, EIM helps companies better understand and manage their information and use it to deliver products faster and at a lower cost than their competitors. The following are some basic questions to ask when considering EIM for your organization.
Q1 – How has EIM technology changed the way Life Sciences organizations do business?
The life sciences business today moves much faster than it did 20 years ago. It’s more competitive, timelines are shorter, and the scope of processes is more diverse and more global. In addition, there is greater need to share SOPs and R&D data across the enterprise. In 1999, quality managers might have walked down to their GMP library and checked out paper SOPs. However today cloud-based systems support a global environment with complex compliance requirements and fast-paced timeline objectives.
Moreover, we know that COVID has presented some unique challenges to the life sciences industry. During the summer of 2021, federal regulators asked a leading pharmaceutical company to dispose of 60 million doses of its coronavirus vaccine due to contamination. The issue appears to have been caused by poor manufacturing processes that are often a result of poor quality management systems and processes.
Q2 – What are some of the challenges with adoption of EIM technologies and how were they overcome?
One of the goals of EIM is to bring information closer to the line of business. Technology puts information into context to enable workers to do their jobs more efficiently. As a result, it literally changes the way people work. And like any other big change, it can present a challenge in terms of change readiness. It is not uncommon for organizations to struggle with prioritizing areas of greatest need, obtaining executive buy-in, and getting workers to appreciate the value of solutions.
The key to success is to create a Change Readiness Plan that is 1) tailored to the change being implemented, 2) focused on transitioning individuals and teams to the desired future state with minimal disruption, and 3) realizes the expected benefits of the change. We specifically use the term ‘Change Readiness’ to differentiate from ‘Change Management’, which is often associated with change control and management of change (MOC) as operational activities. While those elements are important, the human aspects of engagement, reaction management, and goal alignment are correlated more with project success and system adoption.
Q3 – For organizations that have lagged in their digital transformation, where should they start?
One of the best ways to get started with EIM is to adopt a risk-based approach, which prioritizes areas with clear compliance challenges. I often advise my customers to focus initially on a specific problem first with a clear business value and ROI. This is critical to getting executive buy-in early and establishing your champions. Also, early wins enable you to expand your solutions from a place of success. Software Quality Assurance can be a great place to start, followed by the more challenging cases of manufacturing SOPs and batch records.
Q4 – What new innovations are on the horizon?
Process automation, AI, and machine learning will be huge growth areas within EIM over the next 10 years. For many companies, COVID provided a real-life stress test of their processes. Companies that thought they had durable processes realized just how much they were leaning on their workers to keep even simple tasks moving. The goal in the future will be to automate repetitive tasks and let people focus on parts of the process where they provide the most value. This is especially true in the life sciences space, where approval processes, electronic signatures, controlled printing, submission management, and R&D processes are widespread, but traditional workflow implementations have much room for improvement.
Conclusion
EIM solutions for life sciences build on the fundamental insight that business problems are really information problems in disguise. Addressing these problems means understanding the problem, identifying the information needed to solve it, and developing a strategy to turn that information into action. Wildly successful companies like Google, Amazon, and Meta have become the richest companies in the world by collecting and understanding data and maximizing the value of the data they possess. Going forward, for companies to continue to be successful, they must be prepared to do the same by managing and understanding their own data. Life sciences companies are no different.
About Alitek
Alitek is a leader in information based digital transformations. We help organizations improve business outcomes, increase efficiency, and reduce risk, through better management of information. Our experienced team works with you to build a unique strategic plan based on your specific needs, no matter the size of your company.